 Imagine for a moment the worst all-nighter you’ve ever pulled. Now times it by nine. Factor into that the fact that during this all-nighter you have to focus at 100% (or as close to it as is possible), you have to operate and move complex equipment, performing basic algebra and figuring out geometrical problems in reflective space. Congratulations you’ve just completed your first slot of CDI beamtime.
Imagine for a moment the worst all-nighter you’ve ever pulled. Now times it by nine. Factor into that the fact that during this all-nighter you have to focus at 100% (or as close to it as is possible), you have to operate and move complex equipment, performing basic algebra and figuring out geometrical problems in reflective space. Congratulations you’ve just completed your first slot of CDI beamtime.So science is tough. I knew this to begin with, however I didn’t realize just how tough. This week has nearly finished me off with a massive build up of stress, fatigue and disappointment. It’s a good job I’ve hardened myself to the rollercoaster of emotions through years of Ultimate tournaments.
Sadly things did not go our way this time, try as we might. It’s hard to stumble through those last few nights fuelled only by caffeine and pot noodles knowing you are unlikely to get results. We thought we’d prepared well but life always likes to throw us a curve ball from time to time.
I was actually really excited about this beamtime. Not only was I getting to play around with the equipment a lot more but we would be analyzing my sample this time. Or so I thought. Due to some unexpected equipment errors we spent about five days aligning, re-aligning, and then aligning some more. A bit rubbish really. Looks like my date with data was cancelled.
That means it’s back to the drawing board, and I do so love drawing. Or at least I love the scientific process, I’ve usually got a million and one ideas running through my mind and what is great about working here is no one tells you no. The research we are doing is very much frontier work so how can it go wrong when no one knows what is right.
Still planning doesn’t make for exciting blogging unfortunately so instead I’ve decided to dish up two exciting pieces of research from the world of Malaria. One of these has featured in the news quite prominently, and the other is pretty groundbreaking as well.
The first paper relates to the interactions between proteins at the surface of both Plasmodium falciparum merozoite, the parasite responsible for malaria, and red blood cells. These proteins are involved in the invasion of blood cells by this parasite, the subsequent destruction of red blood cells by P. falciparum is what leads to the symptoms of malaria. As this disease kills millions worldwide year on year it is no surprise that falciparum is so intensively studied.
 People have been trying for years to create a new vaccine for malaria however it has been a struggle. One of the big problems is that these proteins at the cell surface show a high degree of redundancy. A protein that is essential for one strain of the parasite to enter a cell will not be for another. This makes it difficult to make one vaccine that is effective for all P. falciparum strains and block malarial infection.
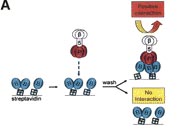
People have been trying for years to create a new vaccine for malaria however it has been a struggle. One of the big problems is that these proteins at the cell surface show a high degree of redundancy. A protein that is essential for one strain of the parasite to enter a cell will not be for another. This makes it difficult to make one vaccine that is effective for all P. falciparum strains and block malarial infection.Enter the cell surface signaling lab at the Wellcome trust Sanger institute. This group have developed a very robust and effective technology for screening extracellular interactions. Here they express simply the external ‘stump’ of these proteins, the bit that sticks outside the parasite cell, as part of a more stable fusion protein. They then probe these with similar constructs of blood cell proteins. By linking this to a protein that can cause a colour change they could discern what binds to what.
What they found was a novel reaction between two proteins, PtRH5 on the parasite, and Basigin on the erythrocyte. This interaction proved critical in invasion, deleting PtRH5 from the parasites genome or blocking Basigin with an antibody almost completely stopped blood cell invasion.
So what’s the most exciting part? Well they tested this on not just one but all the strains of P. falciparum they had available and found the same result. This means produce a vaccine to provide resistance blocking this interaction could provide a reliable preventative treatment for malaria. It’s still early days yet but it is a truly fantastic result.
I am a bit biased of course. I actually worked with the group whom did the main body of the work during my year in industry. I actually remember the main author setting up the screens just before I finished. Interestingly they only worked 9-5 generally as they have a family, proof that it is definitely quality of work not quantity that pays of.
Ok next paper. This is one is also intriguing although I’d say it doesn’t yet have quite the human impact that the previous study does, probably why that one was published in Nature and this was not. This one concerned two topics particularly close to my heart. The cytoskeleton, something I’ve always been interested in, and Sickle cell anemia, because of my African heritage.
Sickle cell anemia is so named because of the odd elongated shape that red blood cells take when they become de-oxygenated. It is a particularly nasty inherited disorder and leads to anemia as well as acute pain due to the bursting of blood capillaries that become blocked by these oddly shaped blood cells.
 The cause of this is a mutation in the protein heamogloblin, the red molecule responsible for binding oxygen in blood cells. The single change in its sequence causes the hemoglobin proteins to clump together making the blood cell much more rigid. Whilst this is a debilitating disease it is recessive, meaning you need a copy of the faulty gene from both your mother and father, to express the symptoms.
The cause of this is a mutation in the protein heamogloblin, the red molecule responsible for binding oxygen in blood cells. The single change in its sequence causes the hemoglobin proteins to clump together making the blood cell much more rigid. Whilst this is a debilitating disease it is recessive, meaning you need a copy of the faulty gene from both your mother and father, to express the symptoms.So how does malaria tie into this? Well if in fact you only have one copy of the gene you do in fact show partial symptoms, not as severe but still somewhat uncomfortable. The upshot however is the added rigidity seems to convey some protection to malarial infection.
Up until now although there are plenty of theories dancing about no one was really sure exactly what the cause of this protection was. That is until a German group looked at the effects of this mutation on the actin cytoskeleton. Actin is one of the proteins that make up a network of fibrous molecules that contribute to the internal structure of cells.
When a red blood cell is invaded by the malaria parasite it’s actin cytoskeleton is reorganized to aid the delivery of a pathogen protein called adhesin to the cells surface. These proteins are, unsurprisingly given their name, responsible for sticking cells to surfaces. The over expression of this protein helps to prevent the destruction of the infected cells by preventing their entry into the spleen.
 Through a powerful microscopy technique known as Electron microscopy these researchers discovered that the critical reorganization of actin in cells expressing the Sickle cell heamoglobin was blocked. This lead to a depletion of adhesion at the red blood cells surface and so they could enter the spleen to be cleared.
Through a powerful microscopy technique known as Electron microscopy these researchers discovered that the critical reorganization of actin in cells expressing the Sickle cell heamoglobin was blocked. This lead to a depletion of adhesion at the red blood cells surface and so they could enter the spleen to be cleared.Whilst this does not have as obvious practical application as the first paper it is an excellent insight into the complex interplay of various cellular components, It’s quite amazing really that even 50 years after the protective phenotype of sickle cell anemia was discovered, only now are we gaining insight into how it works.
Makes me feel better about my lack of results anyway.
Ja, mata ne.




Synchrotron life ... that's how we roll!
ReplyDeleteNice reviews too, especially for a biochemistry-challenged physicist like me!